Spain once again leads clinical trials in Europe, but faces the challenge of competing in the global biomedical race
Our country authorized 962 studies last year, a hundred more than in 2023. Four out of every 10 research projects were dedicated to finding new cancer drugs.
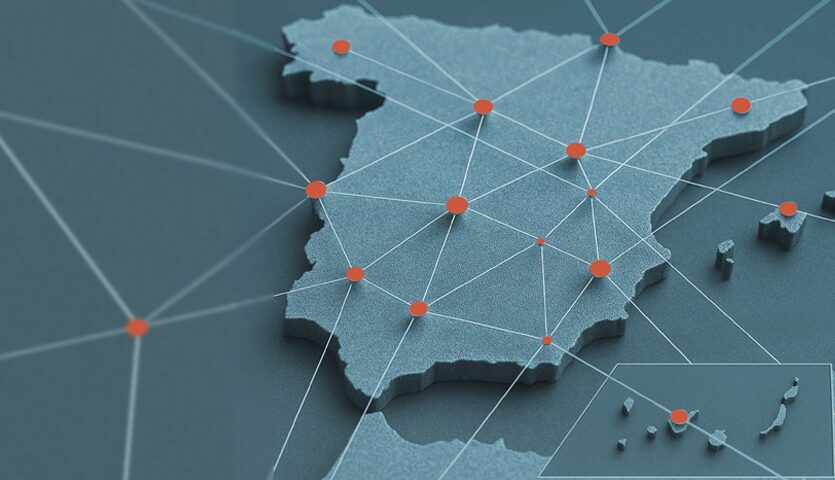
Spain is by far the European country that initiated the most studies in 2025 (849), followed by France (732), Germany (711), and Italy. (593).
Geopolitical changes are transforming the global landscape of health innovation, and Spain and Europe must adopt decisive policies if they want to remain competitive in the global race for pharmaceutical investment.

farmaindustria.es
Last year, Spain authorized 962 clinical trials with drugs, a record number that places our country as the European leader with the highest number of authorized trials, as well as the one that does so in a greater diversity of therapeutic areas. These data are taken from the Spanish Registry of Clinical Studies (REEC), coordinated by the Spanish Agency for Medicines and Health Products (Aemps).
This figure also marks the culmination of a decade of sustained growth in this stage of research, the end of a complex, prolonged, and risky long-distance race that is the R&D of new drugs, which begins when researchers identify a promising compound in the preclinical stage and culminates years later when, if all has gone well, the new drug is made available to patients.

farmaindustria.es
The growth, sustained since 2016, has been particularly evident in the last two years, with more than a hundred additional trials. Furthermore, Spain is by far the most active European country in clinical research. As reflected in the data from the European clinical trials registry platform (CTIS), our country initiated a total of 849 clinical trials in 2025, well ahead of France (732), Germany (711), and Italy (593).
 Clinical Trials Information System (CTIS)
Clinical Trials Information System (CTIS)
farmaindustria.es
The search for new cancer drugs accounts for four out of every 10 trials and is the therapeutic area with the highest number of studies. Spain is the European country with the most research in this area, and several Spanish hospitals are leaders at the European level. These types of studies are key, as highlighted by the Aemps, because they manage to recruit a sufficient number of patients in different countries to obtain solid results and accelerate the development of treatments.
After tumors, the areas with the highest number of studies are immune system disorders (10.5% of research), nervous system disorders (6.9%), cardiovascular disorders (6.2%), and respiratory tract disorders (4.4%). Twenty-two point five percent of trials were devoted to rare diseases.
Phase 1 trials account for 25% and phase 2 trials for 31%, both phases having grown in recent decades, highlighting Spain’s strength in clinical research and the high scientific level of its healthcare professionals, as well as the possibility of offering more options to patients at an earlier stage.
As the Aemps points out, Spain’s leadership is due to several factors. These include a highly skilled hospital network with almost 1,000 centers involved in clinical research over the last five years; growing patient participation and one of the best recruitment rates in Europe; and significant public-private collaboration. It should be noted that more than 80% of clinical trials are funded by the pharmaceutical industry.
The industry’s drive for research is therefore key to Spain and Europe’s ability to continue offering society innovative medicines, especially at a time when the United States and Asia are implementing aggressive trade policies to attract innovation to their territories. This has caused Europe to lose 25% of its R&D share in the last two decades, to the detriment of the US and Asia.
“We have the potential to be leaders in the research, development, and production of new medicines, but geopolitical changes are transforming the global landscape of health innovation, and urgent action is needed to avoid losing the global race for innovation. It is essential to leverage these strengths and take decisive political action so that Europe can remain competitive in the global race for pharmaceutical investment,” emphasizes Juan Yermo, CEO of Farmaindustria.
Making this a reality requires Europe to consolidate and expand the path recently embarked upon to streamline and simplify clinical trial authorization processes, in line with initiatives such as Fast EU and the future Biotech Act, which are necessary to strengthen European competitiveness, attract investment, and, above all, enable European patients to access treatments under development as quickly as possible.
Primary care and accelerated assessment
Over the last year, Farmaindustria has continued to promote new projects to expand the scope of clinical research. One of the most significant of these is the project that seeks to extend research to healthcare centers, which has taken a further step forward this year with the establishment of the new Regional Maps and Observatory for Clinical Research in Primary Care.
The Association has also worked to promote accelerated evaluation procedures for phase I trials, initially intended for advanced therapies and rare diseases and later extended to biological oncology drugs.